Eureka!
Alumni Scientific Discoveries
By Sharon Rippey, Holly Foster and Stacey Himmelberger
From this small liberal arts college on a hill have come the likes of B.F. Skinner '26, one of the most renowned behavioral psychologists, and Paul Greengard '48, whose work in neuroscience earned him a 2000 Nobel Prize. The research conducted by these and other Hamilton alumni -- nine of whom are featured here -- has made a lasting contribution to the world of scientific inquiry.
Hugh Sampson '71
A Big Fight Against a Tiny Enemy
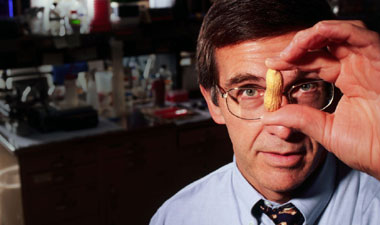 Hugh Sampson '71, director of the Jaffe Food Allergy Institute at Mount Sinai School of Medicine, with his nemesis, the peanut. Photo by Don Hamerman.
Hugh Sampson '71, director of the Jaffe Food Allergy Institute at Mount Sinai School of Medicine, with his nemesis, the peanut. Photo by Don Hamerman.In 1991, pediatric allergist Hugh Sampson '71 had just finished presenting a lecture on food allergies at a scientific meeting in Toronto when he was approached by a woman whose daughter had died of an allergic reaction to peanuts two weeks earlier. "She started asking me questions, and I couldn't give her very many answers," Sampson recalled.
I was thinking 'we should know some of this.' It was an area that was extremely important, and we had virtually no answers," he said.
Sampson went back to Johns Hopkins, where he was practicing at the time, and started looking clinically at peanut allergies. After publishing an article about fatal and near-fatal food-induced anaphylaxis, a foundation expressed interested in supporting research on finding a therapy specifically for peanut allergies.
Now, 12 years after that talk in Toronto, Sampson is chief of pediatric allergy and immunology and director of the Jaffe Food Allergy Institute at Mount Sinai School of Medicine. An acknowledged expert on peanut allergies, his breakthrough work appeared in the summer 2003 issue of the Journal of Allergy and Clinical Immunology (JACI). In October he was elected to the Institute of Medicine of the National Academy of Sciences, whose members have made major contributions to the advancement of the medical sciences, health care and public health. Election is considered one of the highest honors in the field.
An estimated 1.5 million Americans are allergic to peanuts, and each year 50-100 die after contact with some form of the popular food product, according to the Food Allergy and Anaphylaxis Network. Included in the JACI article is Sampson's work as co-author of a study that found doctors, by looking at how a patient's immune system reacts to small bits of peanut protein, can identify patients likely to outgrow their peanut allergies.
"By using a test, we can tell if someone will be reactive to a peanut if they ingest it," he explained. "Based on our early data, we think we can eliminate the need for any kind of challenge test in all but around 10 percent of patients."
Currently the only way to prevent peanut allergic reactions is through education -- reading food labels, avoiding high-risk situations such as foods served in buffets and ice-cream parlors, and unlabeled candy and desserts. If a peanut allergy victim accidentally eats the food, treatment can include injection of epinephrine and a trip to the emergency room. For some peanut allergy sufferers, as little as 1/100th of a peanut can trigger a reaction.
If Sampson's work proceeds as he hopes, prevention of peanut allergy reactions will become a reality. Sampson is leading the work on a new vaccine that will "turn off the allergic response in people with peanut allergies," he said. In the vaccine study, researchers used E. coli bacteria to deliver a vaccine in the form of a suppository that provided effective protection against peanut allergy for up to three months in mice bred to have that allergy.
Sampson said the U.S. Food and Drug Administration has approved the group's plan for human trials. "We are now in the process of getting the vaccine manufactured under all the standards and regulations that are required and hope to start human trials in a year," he said.
Edward Taylor '46
A New Prescription for Fighting Cancer

When the Food and Drug Administration and Eli Lilly & Co. announced that the drug Alimta would be made available to patients with malignant mesothelioma, the news gave hope to thousands suffering from this devastating cancer of the lung lining. The announcement also marked the capstone accomplishment in the distinguished career of Edward Taylor '46, the A. Barton Hepburn Professor of Organic Chemistry Emeritus at Princeton University.
The drug is the result of a 20-year academic-industrial collaboration between Taylor and the pharmaceutical firm Eli Lilly & Co. "Such a long-term collaboration was most unusual, and it resulted in one of the most exciting things I ever experienced in science," Taylor said.
The history of this cancer breakthrough began six decades earlier. Taylor entered Hamilton in the 1940s with the goal of becoming a writer. Since the College required all students to take a science course, he flipped a coin and signed up for chemistry with Dick Sutherland. "In my 60 years of teaching experience, he was the best teacher I've ever encountered," Taylor recalled. "He got me so excited about chemistry that I took every course Hamilton offered. It was Asa McKinney who fired my determination to become an organic chemist."
While he was at Cornell continuing his training, Taylor read an article in the journal Science that reported the discovery of a new growth factor for microorganisms that later became known as folic acid. The bizarre finding that the heterocyclic ring system in this compound was the same as that found in the pigments of butterfly wings motivated Taylor to concentrate on a study of heterocyclic chemistry, and he is now acknowledged as a world expert in this field.
Heterocyclic chemistry deals with ring systems made up of carbon atoms and one or more non-carbon elements. It is a major branch of organic chemistry with broad significance for medicinal chemistry, natural products and organic synthesis.
"When I first worked on the structure of folic acid, I had no idea that it was important. Gradually it became recognized that folic acid is necessary for growth and cell division," Taylor said.
His research at Princeton has focused on the therapeutic potential of inhibitors of folic acid. "The goal is to gain control over unbridled cell growth. The trick was to hit cancer cells rather than normal cells." Taylor spent years looking for ways to disrupt the processing of folic acid in cancer cells without damaging healthy cells.
It was this work that led to Alimta, a drug that blocks several enzymes necessary for cancer cell division and tumor growth. An added benefit is that toxicity from the drug is reduced with folic acid and vitamin B12 supplements. In addition to compassionate use (which refers to providing a drug to a patient on humanitarian grounds before the drug has received official approval) in the treatment of mesothelioma, Alimta has also shown striking activity against such solid tumors as breast, colon, lung, pancreas and bladder cancers.
Although he never became a best-selling author, Taylor has published more than 450 scientific papers, was author or editor of more than 80 volumes on organic and heterocyclic chemistry, has more than 50 U.S. patents on organic synthesis and medicinal chemistry, and is now the inventor of a compound that fights cancer.
Michelle Walvoord '94
Unearthing Nitrogen in the Desert
 Michelle Walvoord '94 collecting samples in the Nevada Amargosa Desert.
Michelle Walvoord '94 collecting samples in the Nevada Amargosa Desert.While a Ph.D. candidate at the New Mexico Institute of Mining and Technology, Michelle Walvoord '94 and University of Reno hydrologist Peter Hartsough were working in the Nevada desert, studying chloride in the subsoil. During a routine gathering of core samples, something unexpected happened -- they discovered large pools of previously undetected nitrogen reserves several meters below the surface. Their finding raises new questions about desert ecosystems.
A paper detailing the discovery, with Walvoord as first author, was published in the Nov. 7, 2003, issue of the journal Science. "The gist of the Science magazine paper is that we've come upon a source of nitrogen that is potentially a very large component of the global soil nitrogen budget," said Walvoord, now a National Research Council postdoctoral associate for the U.S. Geological Survey in Lakewood, Colo.

Michelle Walvoord '94 conducting
research in Alaska
According to Walvoord, the nitrogen reserves have two potential implications: first, groundwater nitrate can cause serious contamination if Southwestern deserts are irrigated or the climate becomes wetter. High nitrate concentrations in drinking water have been linked by the Environmental Protection Agency to a blood disease, miscarriages and non-Hodgkins lymphoma. There are also ecological implications because plants in desert systems are thought to be nitrogen-limited. "The soils there are poor in nitrogen, but below the root zone is this huge pool of nitrogen in the form of nitrate that we've discovered. That's going to cause ecologists to rethink how desert systems work," she said.
Walvoord became interested in hydrology at Hamilton. "Todd Rayne (associate professor of geology) exposed us to hydrology as a subfield of geology. I had a few talks with him about next steps in a career," she recalled. "I also spent a semester abroad in Tanzania where I worked on a project to evaluate the effects of sewage pollution on the coral reefs near Zanzibar. My education and background at Hamilton provided a foundation for what I'm doing today," she said.
As a Ph.D. candidate, Walvoord discovered her research niche -- studying water in dry desert areas. "These types of environments are important from a hydrologic perspective because of the increasing demand for already stressed water supplies due to rising populations. The desert systems hadn't been looked at much from a hydrology standpoint, so people didn't know how quickly the groundwater is being replaced," she explained. "It wasn't until the past few decades that a lot of people have been moving into arid regions and been using a lot of water. There's a big push to see how much water is out there and how much is being used."
Walvoord said desert environments are also being targeted for hazardous waste disposal because the groundwater there is deep and because there's no water percolating into the system to transport contaminates into the drinking water supply. "Yet there are still so many gaps in knowledge in terms of how water and contaminants move through these dry systems," she added.
Walvoord's research on desert vegetation promises to challenge more assumptions about plants in the desert. "Plants are so important to the water balance," she said. "We can't understand hydrology without understanding ecology."
Stanley Kaye '74
The Power of Fusion

In the movie back to the future, a blender-sized fusion-powered electric generator powers the time-traveling DeLorean back to the 1950s. Stanley Kaye '74, principal research physicist at the U.S. Department of Energy's Princeton Plasma Physics Laboratory, Princeton University, admits the technology he's developing won't make time travel possible, but it may provide enough gigawatts to power an entire city.
Kaye, who is known internationally for his work in magnetic confinement fusion, is developing methods of producing energy by fusing together light elements, such as hydrogen. This is the same process that powers the sun.
"We create a strong magnetic field inside a device called a tokamak," he explained. "The magnetic field can be considered to be a magnetic 'bottle' that is used to contain an ionized gas, usually hydrogen, called a plasma. We heat the plasma, and, when hot enough, the protons fuse together and produce energy."
The goal is to keep the plasma hot, but Kaye has found that the magnetic bottle has "holes" that allow heat to seep out. He and his team of researchers have been working to understand the processes that cause this loss of energy.
In 1999 the Department of Energy built a tokamak with a spherical design developed by a team co-headed by Kaye. The new design, according to Kaye, was a product of theorists and experimentalists working together. "Theory told us that this design should lead to improved performance, and we developed a design that we believed would take maximum advantage of this predicted benefit. Of course the real proof is how well the device actually operates, and after three years we can report that there have been dramatically good results."
Before commercial fusion energy can become a reality, there are hurdles to overcome. "The physics and technology are challenging. We not only have to understand the physics, but we have to build equipment and develop the materials that can withstand the heat generated by sustained fusion processes," Kaye said. "Harnessing fusion energy requires a combination of scientific and engineering advances."
Recently, there has been increased funding to explore alternative energy sources, and fusion is getting renewed support. "Fusion power generation would not have the daily weather-related variations or the extended power transmission issues faced by solar and wind power, also viable alternative energy options," Kaye said. "Once it's developed -- and it is still decades away from practical application -- it will be possible to have fusion power plants anywhere. It is an attractive source of energy in that the source of fuel (hydrogen) is plentiful, and in that it's relatively clean -- not like fission which has the problem of nuclear waste."
Fusion is not like installing solar panels on your house; in order to make it cost-competitive, the fusion reactors would need to be built to power a region or city. "The question is, how much more are we willing to pay out of pocket to protect the environment," Kaye said. "If we don't invest in alternatives like fusion energy, we'll pay the severe price that burning fossil fuels will have on our environment."
Marcy Kingsbury '93
Making Bigger, Smarter Brains

Marcy Kingsbury '93 and a team of scientists at The Scripps Research Institute in La Jolla, Calif., recently made a discovery that unfolded -- or in this case folded -- in a matter of hours right before their eyes.
A postdoctoral researcher whose work focuses on brain development, Kingsbury and her group study the effects that phospholipids (molecules of fat) have on developing brains. They found that one phospholipid called lysophosphatidic acid (LPA) can act as a signal that stimulates neurogenesis -- the formation of new neurons -- by activating an LPA receptor in the brain. Previously scientists believed that growth factors and other proteins largely controlled neural development and neurogenesis.
Results of their findings appeared in the December 2003 edition of the journal Nature Neuroscience. Kingsbury is the lead author of the article, "Enhanced Cerebral Cortical Growth and Folding by Non-Proliferative Effects of Lysophosphatidic Acid."
In the lab, Kingsbury studied the brains of embryonic mice. One hemisphere of the brain was activated with LPA; the other half observed in a controlled condition. "LPA works by stimulating folds in the brain's cortex," she explained. "Although mammals with large brains, like humans, have these cortical folds, mammals with smaller brains, like mice, have cortex that is smooth. These folds in larger brains arise due to the increased surface area of the brain that is believed to be linked to sophisticated functions such as intelligence and reasoning. Our voil?! moment came when we realized that, by using LPA, we were able to cause massive folding and increased growth of the cortex in an unprecedented 17 hours' time.
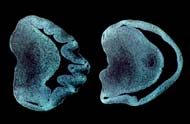
Cortical hemispheres of a brain treated
without LPA (left) versus with LPA (right).
"At first, we thought the increased neural growth came as a result of the brain hemisphere actually collapsing, but, no, this was definitely something specific to LPA," she added.
Furthermore, Kingsbury's team found that the increase in neural cells happened not by cells multiplying, as is common for other cells exposed to LPA, but instead by a new mechanism that helps cells that would normally die stay alive and prematurely divide to form young neurons.
Kingsbury's work may pave the way to better understanding neural diseases -- such as certain types of schizophrenia -- that arise from developmental defects as the brain is setting itself up. "The work may also help us understand how to promote stem cell development, an important step for stem cell therapy," she added.
A psychobiology major at Hamilton, Kingsbury completed her Ph.D. at Cornell University in 2000 before joining Scripps. She plans to continue investigating the factors that can affect the brain, particularly how molecules, such as lipids, can change brain development and what this might mean for behavior of the organism. One question she hopes to answer one day -- are these changes in the brain beneficial, or do they predispose the organism to disease?
Jonathan Overpeck '79
A Forecast for the World

Jonathan Overpeck '79 takes a break to enjoy the scenery while collecting sample sediments in Panch Pokari, a high alpine lake in Nepal not far from Mount Everest.
Jonathan Overpeck '79 likens the thousands of miles of ice that make up the Arctic ice cap to the proverbial canary in the mineshaft. "Every part of the Arctic is changing in an unprecedented way," said Overpeck, director of the Institute for the Study of Planet Earth at The University of Arizona. "It is the harbinger of what's coming for the entire globe. It's serious."
Looking at thermometer records from the last century, there is little doubt that the earth has warmed. The year 1998 was the warmest on record, with 2001 coming in second and 2003 likely close behind. Overpeck, also a professor of geosciences and one of the leading experts on climate change, said, "There is consensus among climate change scientists that warming is global, and related, at least in part, to humans. The real concern is how much more the earth will warm (estimates range from 1.4 and 5.8 degrees C by 2100), how fast it will warm and what the impacts will be."
A paleoclimatologist by training, he has traveled much of the globe to study how Earth's environment works. His most significant discovery -- that the Arctic is now warmer than it has been in centuries -- was published in the journal Science. More recent reports of sea ice retreat are a clear sign that the Arctic, including the Greenland Ice Sheet, may be on the verge of abrupt melting.
To gather his data, Overpeck created a synthesis of Arctic environmental variability over the last 400 years by examining the climate record in tree rings, for example, as well as changes in lake sedimentation. He also assembled information from ice cores, looking for evidence of when previous summer melting occurred. Then he and his team determined how changes in "climate forcing," such as increased carbon dioxide or solar and volcanic variability, likely caused the observed temperature changes. More recently completed comparisons between observed and computer-modeled climate change have led Overpeck to forecast future temperature conditions.
"Until now, most scientists thought ice sheets would take many thousands of years to melt," Overpeck said. He explained that if sea ice around the polar regions starts melting, it would allow more radiation to warm the earth. This radiation will be absorbed by the ocean, rather than being reflected out to space. The rate of sea ice retreat would then control how fast the polar regions warm.
"Greenland Ice Sheet melting is a perfect example of what might be in store for us," Overpeck said. "Our research using the paleoclimatic record shows evidence that it has melted in the past, and future melting could be much faster than first anticipated. It could end up wiping out whole countries (island nations) with the associated sea level rise. The heavily developed coasts of the United States would be hit hard, too."
Overpeck has been working to get this information in front of the scientific community to make sure everyone understands the implications of human-caused climate change. Asked if he considers himself an environmental activist, he said, "My main goal is to make sure people in society, from Congress down to individual voters, have a good scientific basis on which to base their decisions rather than just special-interest or political science."
Thomas Ducibella '69
Creating Happy Endings
 For every couple whose dreams of having a baby come true thanks to in vitro fertilization (IVF), two other sets of would-be parents spend thousands of dollars trying to conceive with no success. Tom Ducibella '69, professor of obstetrics and gynecology at Tufts University, is working to better those odds.
For every couple whose dreams of having a baby come true thanks to in vitro fertilization (IVF), two other sets of would-be parents spend thousands of dollars trying to conceive with no success. Tom Ducibella '69, professor of obstetrics and gynecology at Tufts University, is working to better those odds.
Ducibella has been studying mammalian embryos and problems associated with IVF since 1973, five years before the first "test-tube baby" was born. "In my teaching and research, fertilization is my favorite topic," he said. "It initiates development of entire organisms."
The IVF procedure works like this: multiple hormone-induced eggs are recovered from the patient then placed with the sperm in a nutrient mixture inside a culture vessel. The eggs are inseminated as well as monitored during the process of fertilization and early cell division, which would normally occur in the fallopian tubes. The cleaving embryos are then placed into the uterus a few days later.
Ducibella studies the biochemical factors that may contribute to lower fertilization. His current research explores the critical role calcium plays in the process. "The release of calcium within the egg is necessary for most, if not all, of the major events of egg activation that are responsible for initiating the development of the embryo," he said. His lab has found that calcium does not simply act as a single "on/off switch" but in a series of pulses which regulate the expression of proteins, the cell (division) cycle and secretion. These findings were highlighted in an editorial in the journal Science in 2002.
"Different steps of the fertilization process have distinctive calcium requirements," Ducibella explained. "Calcium passes the baton to specific proteins, which in turn stimulate the completion of meiosis, cell division (cleavage) and synthesis of embryonic proteins, including preventing multiple sperm from penetrating the egg (which causes the zygote to die). It's like Morse code, and calcium is the signal."
Although getting into the field of in vitro fertilization was a matter of "being in the right place at right time" -- the lab where he was doing his training was working on sperm development, fertilization and pre-implantation development -- Ducibella credits Hamilton with his success as a researcher.
"(Professors) Robin Kinnel and Duncan Chiquoine went beyond the textbook facts into the 'whys' and 'hows' of embryonic development and the structure of the organic universe," he said. "Writing in virtually all courses prepared me for scientific writing ... which is a main part of what scientists do. You might have a great idea, but if you can't communicate it well, it won't get funded." He also uses his French to e-mail an important research collaborator in Paris.
Ducibella's work has been supported by the National Institutes of Health for the past 20 years. He also participates in a NIH study section, reviewing grant proposals with his friend, William Wright '71, at Johns Hopkins. "What are the chances that a couple of people who went to a small liberal arts college end up on the same NIH study section?" Ducibella added. "That says something about Hamilton."
Richard Edelson '66
A Search-and-Destroy Cancer Weapon
 When Richard Edelson '66 was named director of the Yale Cancer Center in June, he was described as "a world-class investigator" by David Kessler, dean of the Yale Medical School. Edelson will put those investigative skills to the test when trials begin on a new treatment that could be a breakthrough in fighting lung cancer.
When Richard Edelson '66 was named director of the Yale Cancer Center in June, he was described as "a world-class investigator" by David Kessler, dean of the Yale Medical School. Edelson will put those investigative skills to the test when trials begin on a new treatment that could be a breakthrough in fighting lung cancer.
Edelson is internationally known for his work in immunotherapy as a treatment for cancer. A professor and chair of dermatology at Yale, he describes immunotherapy as "using the power of the immune system to fight a cancer, to destroy or control its growth."
The Yale School of Medicine graduate is credited with developing a widely used treatment for cutaneous T-cell lymphoma -- a potentially fatal disease caused by malignant T lymphocytes that initially affect the skin before disseminating throughout the body. Edelson and his research team devised and implemented the first FDA-approved selective immunotherapy for any cancer, a treatment now called transimmunization.
"Active immunotherapy is like a vaccination against cancer. You're trying to stimulate the patient's own immune system to fight its own battle," Edelson said. "Typically, we think of vaccination as a means of preventing a disease from starting. It is much more challenging to immunize a patient against a cancer that has already established a foothold in the body, but that is the reality and the necessity."
Pending approval by Yale's human investigation ethics committee, Edelson and the Yale Cancer Center team are planning to conduct clinical trials in 20 lung cancer patients, each with an advanced form of malignancy. Through transimmunization, extracts of a patient's cancerous cells are combined with his or her normal cells to form a "cellular vaccine." This personalized vaccine can then be administered by simple injection. "By stimulating the patient's own T cells to better respond to the cancer, an ongoing immunologic attack against the malignant cells can be initiated. At their best, these vigilant anti-cancer T cells have much better vision than we clinicians do. They can find and destroy the Ôneedle in the haystack' -- microscopic collections of residual cancer cells capable of expanding lethally," Edelson explained.
The researchers initially developed immunotherapy as a way of stimulating a chemotherapeutic drug by means of a light. By passing a leukemia patient's blood outside the body, through a special device in the drug that was activated by light, the leukemic cells were damaged in a way that caused them to die within a few days. When the damaged cancer cells were returned to the patient's body, the researchers were astonished to discover that even the leukemic cells that had not been exposed to the drug-activating light disappeared in some patients.
"It became clear that if we could accomplish that by exposing only five percent of the patient's cancerous cells to this light-activated drug, then somehow we were immunizing the patient against the cancer. The immune system was being educated to search and destroy the remaining cancer," Edelson said.
Since receiving FDA approval in 1988, immunotherapy has been administered some 250,000 times in 150 medical centers around the world. "Transimmunization was a serendipitous clinical advance waiting to be understood," Edelson said. "That hard-earned understanding now in hand, we have refined the treatment and are optimistic about its potential efficacy in a broad range of cancers."
Barry Kreiswirth '76
The Disease Detective

Criminal investigators commonly use DNA fingerprinting as forensic evidence. Barry Kreiswirth '76, director of the Public Health Research Institute Tuberculosis Center, applies the same technology to identify diseases.
"Just as people have a unique fingerprint, we can identify a small portion of bacterial DNA, known as a DNA marker, to identify a unique strain of a disease," he said.
At Hamilton, Kreiswirth studied electron microscopy. He then become a technician in a breast cancer clinic before going back to school. "I saw you couldn't make a career out of a 'technique' because technology is always evolving." he said. "Technology is the underlying engine, but you have to understand the science or else all you have is a bunch of data and no answers."
While Kreiswirth was completing his training at New York University, a new disease was mystifying the medical community. His laboratory had acquired a collection of Staphylococcus aureus isolates recovered from cases of Toxic Shock Syndrome associated with tampon use. He worked to identify, clone and genetically characterize the causative toxin for his Ph.D. dissertation. "I worked from the beginning of identifying a new health problem to characterizing the genetic factor and understanding how it causes disease," he said. "Once you know this process, you can characterize any emerging pathogen, like, for example, SARS or West Nile."
At the Public Health Research Institute, in collaboration with the New York City Health Department, he put this training to use when tuberculosis reemerged in epidemic proportions. According to Kreiswirth, TB had been under the radar screen for several years but became a serious problem with HIV co-infection and the emergence of multi-drug resistant strains.
Using DNA fingerprinting technology, Kreiswirth identifies specific TB strains or genotypes. "We use single nucleotide polymorphisms. The nucleotide changes have proven to be a powerful method to distinguish the species into a genetic tree," he said. "We are currently mapping suspected virulence determinants along this evolutionary tree to understand the pathogenic make-up of M. tuberculosis." To date his lab has genetically characterized some 17,000 strains of TB, more than 3,000 of which are multi-drug resistant.
In addition to the genotyping, Kreiswirth relies on computer technology to track where strains of the disease spread. Each TB strain has a barcode that, like a fingerprint, gives specific data. Kreiswirth worked internationally to create surveillance and research collaborations. "If you get TB, we can tell you what strain you have and where that type originated," he explained. "One of my projects is fingerprinting TB among Siberian prisoners. When that particular strain showed up in New York City, we knew that Russian immigrants were bringing it with them."
Kreiswirth's work describing the multi-drug resistant strain "W" that caused the M. tuberculosis outbreak in New York City and its relationship to the global dissemination of strains throughout Asia and Russia has been published in such journals as JAMA, the Journal of Infectious Diseases and The New England Journal of Medicine. Although internationally recognized in the field, Kreiswirth admits his investigative public health work will never be done. "Did you know that hospital-acquired infections kill tens of thousands of people every year in the U.S.? It's a major medical problem because the bacteria in hospitals are becoming multi-drug resistant."
